Made in Bavaria – for the world
„Menschenleben retten aus Überzeugung.“
Der LifesafAIR® des Ingolstädter Unternehmens trimatec wurde bedingt durch die Corona-Pandemie im März 2020 von diversen Spezialisten aus Medizin, Medizintechnik, Softwareentwicklung, Qualitätsmanagement und agilem Projektmanagement in Rekordzeit entwickelt. Das Gerät befindet sich aktuell im Konformitäts-Bewertungsverfahren, die CE-Zertifizierung nach Medizinprodukte-Verordnung (EU) 2017/745 wird demnächst erwartet.
Aus der Motivation des Projektteams: „Wenn wir nur ein Menschenleben retten, dann hat es sich gelohnt!“ wurde ein großes Ziel.
Das Produkt
Anfangs nur für die Corona-Pandemie konzipiert ist der LifesafAIR® zum Intensiv-Beatmungsgerät weiterentwickelt worden. Innerhalb kürzester Zeit können mittels der patentierten Bauweise Intensiv-Beatmungsgeräte in großer Stückzahl produziert werden. Dies wird durch die durchgängige Verwendung von Hochleistungs-Industriebauteilen ermöglicht welche auf dem Markt in hoher Stückzahl verfügbar sind. Es wurden keine Abstriche bei der Funktionalität gemacht. LifesafAIR® ist zur Behandlung Schwerkranker während aller Phasen eines schweren Lungenversagens (ARDS) einsetzbar.

Equipped to save lives.
Die Bauweise unseres LifesafAIR®
Extrem ROBUST
Der LifesafAIR® ist robust, um extremen Klimabedingungen standzuhalten, und kann unter dem Einfluss rauer Umweltfaktoren betrieben werden. Er ist für die invasive und nicht-invasive Beatmung konzipiert. Das effiziente Batteriesystem ermöglicht einen Betrieb ohne Stromversorgung, optional auch für mehr als 2 Stunden.
Unglaublich ANWENDERFREUNDLICH
Für den LifesafAIR® ist die einfache und benutzerfreundliche Struktur und Steuerung der Schlüssel, um so vielen Menschen wie möglich High-Tech-Beatmung zur Verfügung zu stellen. Die geprüften und zertifizierten Standardkomponenten sowie die Fernwartung machen das Gerät auch auf Dauer erschwinglich und wartungsarm.
Made (and Engineered) in Germany
Für die Entwicklung des LifesafAIR® haben wir branchenübliche Komponenten verwendet die in unzähligen Hightech-Maschinen der internationalen Industrie erfolgreich eingesetzt werden.
Das Herzstück des Beatmungsgeräts vereint das Wissen und die Erfahrung aus Jahrzehnten deutscher Ingenieurskunst.
Leistungsmerkmale
Die Bandbreite unseres LifesafAIR®
Geeignet für invasive Beatmung (Tubus)
(druck- oder volumenkontrolliert), mit oder ohne Unterstützung der Spontanatmung
Geeignet für nicht invasive
Beatmung (Maske oder Nasenbrille)
Zusätzlich mit High-Flow-Oxygenisierung
Einfache Wartung
Dank der Verwendung von Industriebauteilen und Verzicht auf Sonderteile ist eine simple Wartung und unproblematische Ersatzteilversorgung gewährleistet. Alle Bauteile sind einfach und schnell zugänglich.
Features
Zur Beatmung von Patienten ohne eigene Spontan-Atmung bei Verwendung eines Tubus oder Maske. Druck und Sauerstoffgehalt sind dabei stufenlos regelbar von 5…80mbar bzw. 21%…100% Sauerstoff. Ein einstellbarer PEEP-Druck sorgt
dafür dass beim Ausatmen der Mindestdruck nicht unterschritten wird.
Zur Beatmung von Patienten ohne eigene Spontan-Atmung bei Verwendung eines Tubus oder Maske. Volumen und Sauerstoffgehalt sind dabei stufenlos regelbar von 50…2.000ml bzw. 21%…100% Sauerstoff. Ein einstellbarer PEEP-Druck
sorgt dafür dass beim Ausatmen der Mindestdruck nicht unterschritten wird.
Zur Beatmung von Patienten mit eigener Spontan-Atmung bei Verwendung einer Maske. Der Sauerstoffgehalt ist dabei stufenlos regelbar von 21%…100%. Ein einstellbarer PEEP-Druck sorgt dafür dass beim Ausatmen der Mindestdruck
nicht unterschritten wird.
Bei einer nicht-invasiven Behandlung (Nasen-Kanüle oder Maske) ist eine High-Flow Sauerstoff-Behandlung möglich. Flow und Sauerstoffgehalt sind dabei stufenlos regelbar von 1…80 l/min bzw. 21%…100% Sauerstoff.
Bei Einsatz dieses Modus ist eine nachgeschaltete Atemgas-Temperierung und -Anfeuchtung erforderlich!
Vorhandenes Equipment mit ISO-Anschluss Ø 22mm kann verwendet werden, keine Adaption erforderlich!
über 10“-Touchscreen, minimaler Schulungsaufwand.
Zentrale Gasversorgung oder Gasflaschen-Verbindung mit DIN-Konnektoren (DIN 13260), auf Nachfrage auch MEDAP oder andere Anschlussarten möglich.
Patienten-Zweischlauch-System Ø 22mm konisch mit Codierung über Innen- und Außendurchmesser, handelsübliche Filter und Patienten-Schlauchsysteme anschließbar.
Elektrische Versorgung 110/230VAC 50/60Hz, Anschlusskabel für alle Länder-Varianten erhältlich.
Fahrbar mit schwenk- und feststellbaren Rädern.
Autarke Energieversorgung über Akku.
Flaschenhalterung für 5kg-Flasche (Ø 140mm), Sauerstoff- oder Luftmenge ausreichend für circa 2 Stunden autarke Beatmung.
Instant-On bei Überbrückung des Geräte-Selbsttests: 50sek. vom spannungslosen Gerät bis zum Start der Beatmung!
Integrierte Not-Stromversorgung für Stromausfälle bzw. Transport mindestens 30 Minuten.
+ mit optionalem XL-Akkupaket Netzunabhängigkeit für mindestens zwei Stunden.
Mehrfarbige LED zur Signalisierung der Betriebszustände.
Optische und akustische Alarmierung.
Ergonomischer Tragarm (dreh-/schwenk-/neigbar).
Robustes, stabiles Metallgehäuse, Langzeit-Lagerungstauglich, „Rugged-Use“.
+ Optionales Online-Konnektivitäts-Paket: Remote-Software-Updates, Remote-Unterstützung (Telemedizin, Fernwartung).
Keine Kosten bei Patientenwechsel durch Verbrauchsmaterialien.
Durch Beatmung kontaminierte Bauteile können innerhalb von wenigen Minuten ausgebaut und in einem Autoklaven sterilisiert werden.
Einstell-Möglichkeiten
Atemfrequenz
2 … 100 Atemzüge pro Minute
Atemvolumen
50 … 2.000 ml pro Atemzug
I/E-Verhältnis
1:0,5 … 1:4
PEEP
0… 50 mbar
Pinsp
0… 70 mbar
Patientengetriggerte Atmung
0… 10 mbar


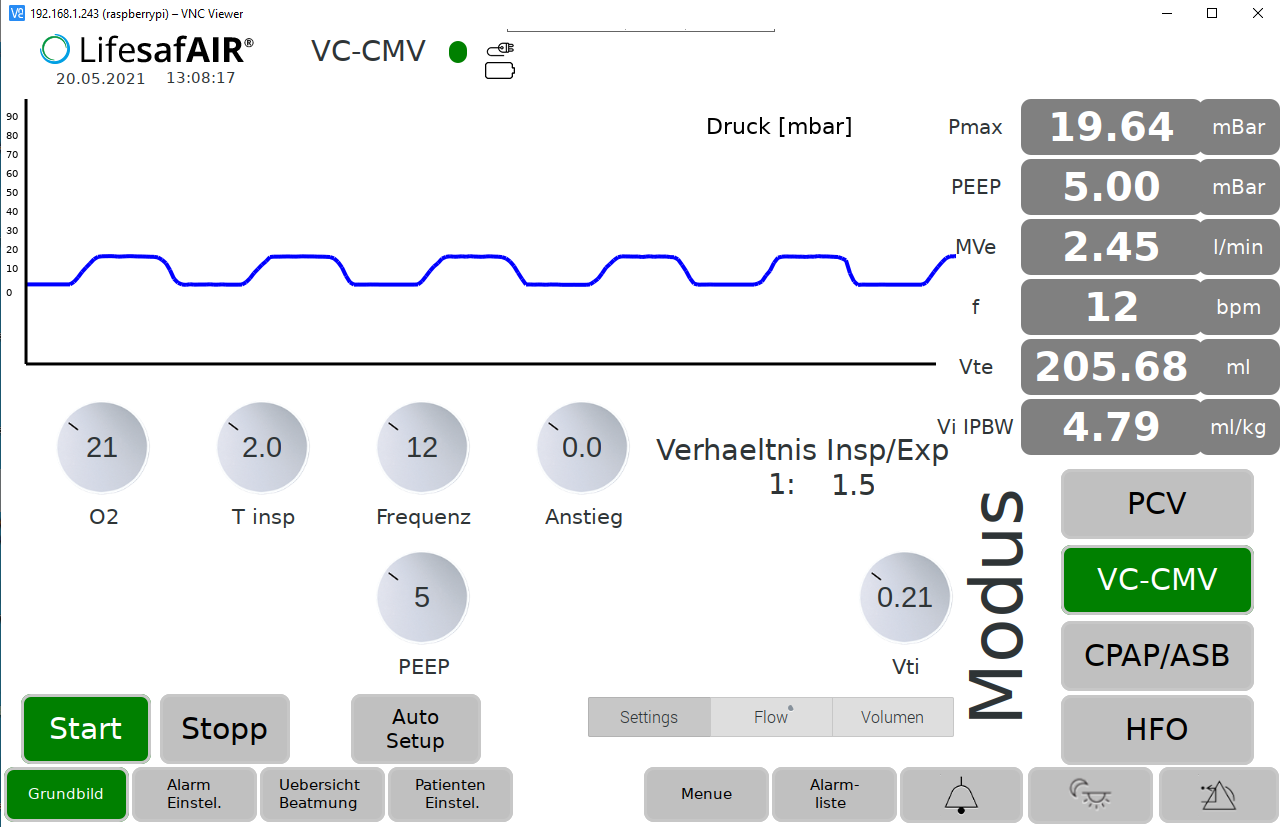
FiO2
21 … 100% Sauerstoffgehalt
Apnoe-Zeit
5 … 60 Sek
Meereshöhe
bis 2.000 m
Patienten-Alter
ab 2 Jahre
Patienten-Gewicht
ab 10 kg
Patienten-Größe
ab 80 cm
Gewicht: 85 kg
Abmessungen: Breite 730 mm, Tiefe 349 mm, Höhe 1.063 mm
Inspiratorischer Anschluss (Einatem-Schlauch): ISO 22 mm (OD) konisch
Exspiratorischer Anschluss (Ausatem-Schlauch): ISO 22 mm (ID) konisch
Anschluss Luft-Versorgung: kodiert nach DIN13260, max. 8 bar
Anschluss Sauerstoff-Versorgung: kodiert nach DIN13260, max. 8 bar



