Made in Bavaria – for the world
“Saving lives out of conviction.”
The LifesafAIR® of the Ingolstadt-based company trimatec was developed in record time by various specialists from medicine, medical technology, software development, quality management and agile project management due to the corona pandemic. The device is currently in the conformity assessment process; CE certification in accordance with Medical Device Regulation (EU) 2017/745 is expected soon.
The motivation of the project team: “If we only save one human life, it will be worth it!” Became a major goal.
The product
Initially designed only for the corona pandemic, the LifesafAIR® has been further developed into an intensive care ventilator. Thanks to the patented design, intensive ventilation devices can be produced in large numbers within a very short time
to be produced. This is made possible by the consistent use of high-performance industrial components which are available in large numbers on the market. No compromises were made in terms of functionality. LifesafAIR®
can be used to treat seriously ill patients during all phases of severe lung failure (ARDS).

Equipped to save lives.
The construction of our LifesafAIR®
Extremely ROBUST
The LifesafAIR® is robust to withstand extreme climatic conditions and can be operated under the influence of harsh environmental factors. It is designed for invasive and non-invasive ventilation. The efficient battery system
enables operation without power supply, optionally for more than 2 hours.
Incredibly USER FRIENDLY
For the LifesafAIR®, the simple and user-friendly structure and control is the key to making high-tech ventilation available to as many people as possible. The tested and certified standard components
as well as remote maintenance make the device affordable and low-maintenance in the long run.
Made (and Engineered) in Germany
For the development of the LifesafAIR®, we have used components customary in the industry, which are successfully used in countless high-tech machines in international industry. The heart of the ventilator united
the knowledge and experience from decades of German engineering.
Performance characteristics
The range of our LifesafAIR®
Suitable for invasive ventilation (tube)
(pressure or volume controlled), with or without the support of spontaneous breathing
Suitable for non-invasive
Ventilation (mask or nasal cannula)
Additionally with high-flow oxygenation
Easy maintenance
Thanks to the use of industrial components and the elimination of special parts, simple maintenance and the unproblematic supply of spare parts are guaranteed. All components can be accessed quickly and easily.
Features
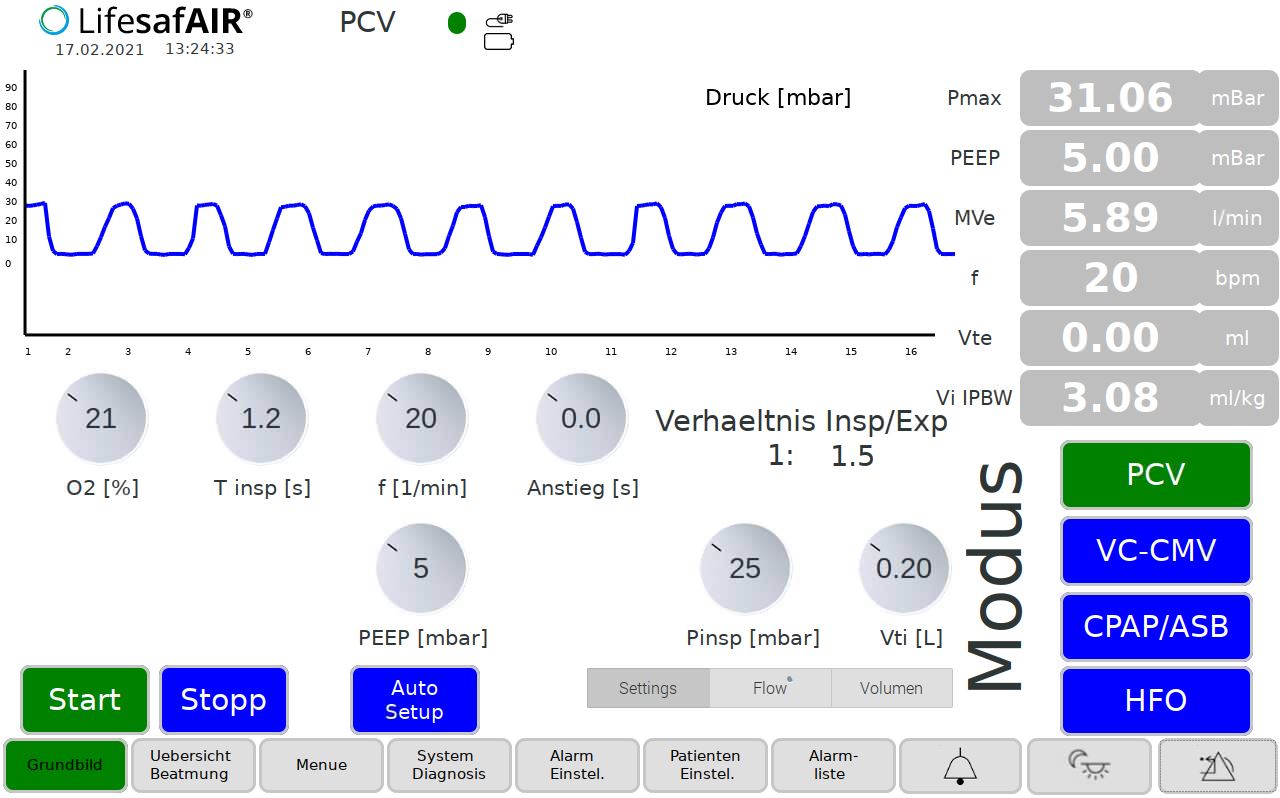
Pressure-controlled ventilation (PCV mode)
For ventilation of patients without their own spontaneous breathing when using a tube or mask. The pressure and oxygen content are continuously adjustable from 5 … 80 mbar or 21 %…100% oxygen. An adjustable PEEP pressure ensures
to ensure that the minimum pressure is not fallen below when exhaling.
Volume controlled ventilation (VC-CMV mode)
For ventilation of patients without their own spontaneous breathing when using a tube or mask. Volume and oxygen content are continuously adjustable from 50 … 2,000 ml or 21 %…100% oxygen. An adjustable PEEP pressure
ensures that the pressure does not fall below the minimum when exhaling.
Spontaneous breathing support (CPAP / ASB mode)
For ventilation of patients with their own spontaneous breathing when using a mask. The oxygen content is continuously adjustable from 21 %…100% . An adjustable PEEP pressure ensures that the minimum pressure when exhaling
is not fallen below.
High-flow oxygen therapy (mode HFO)
In the case of non-invasive treatment (nasal cannula or mask), high-flow oxygen treatment is possible. Flow and oxygen content are continuously adjustable from 1 … 80 l / min or 21 %…100% oxygen.
When using this mode, a downstream breathing gas temperature control and humidification is required!
Filter system, humidifier and anesthetic gas equipment can be attached externally
Existing equipment with ISO connection Ø 22mm can be used, no adaptation required!
connections
Central gas supply or gas bottle connection with DIN connectors (DIN 13260), MEDAP or other types of connection possible on request.
Two patient hose system Ø 22mm conical with coding of inner and outer diameter, commercially available filters and patient hose systems can be connected.
Electrical supply 110 / 230VAC 50 / 60Hz, connection cable available for all country variants.
Mobile and quick to use
Mobile with swiveling and lockable wheels.
Self-sufficient energy supply via battery.
Bottle holder for 5kg bottle (Ø 140mm), oxygen or air quantity sufficient for approx. 2 hours of self-sufficient ventilation.
Instant-On when bridging the device self-test: 50sec. from the de-energized device to the start of ventilation!
Integrated emergency power
Integrated emergency power supply for power outages or transport for at least 30 minutes.
+ With the optional XL battery pack, it is independent of the mains for at least two hours.
Simple and ergonomic handling
Multi-colored LED for signaling the operating status.
Optical and acoustic alarms.
Ergonomic support arm (rotatable / pivotable / tiltable).
Robust, stable metal housing, suitable for long-term storage, “rugged use”.
+ Optional online connectivity package: remote software updates, remote support (telemedicine, remote maintenance).
Easy disinfection and sterilization. All over.
No costs when changing patients due to consumables.
Components contaminated by ventilation can be removed within a few minutes and sterilized in an autoclave.
Setting options
Respiratory rate
2 … 100 breaths per minute
Respiratory volume
50 … 2,000 ml per breath
I / E ratio
1:0,5 … 1:4
PEEP
0 … 50 mbar
Pinsp
0 … 70 mbar
Patient-triggered breathing
0 … 10 mbar


FiO2
21 … 100% oxygen content
Apnea time
5 … 60 sec
Sea level
0 … 5,000 m
Patient age
from 2 years
Patient weight
from 10 kg
Patient size
from 80 cm
Weight: 85 kg
Dimensions: width 730 mm, depth 349 mm, height 1,063 mm
Inspiratory connection (inhalation tube): ISO 22 mm (OD) conical
Expiratory connection (exhalation tube): ISO 22 mm (ID) conical
Air supply connection: coded according to DIN13260, max. 8 bar
Oxygen supply connection: coded according to DIN13260, max. 8 bar



